Sf4 Lewis Structure Molecular Geometry
1 needs to know some basic properties of the given compound and its Lewis structure to empathise its molecular geometry, polarity, and other such properties. SF4 is a chemic formula for Sulfur Tetrafluoride. Information technology is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather chancy compound but is used widely in chemical and pharmaceutical companies.
| Proper noun of molecule | Sulfur Tetraflouride ( SF4) |
| No of Valence Electrons in the molecule | 34 |
| Hybridization of SF4 | sp3 hybridization |
| Bond Angles | 102 degrees and 173 degrees |
| Molecular Geometry of SF4 | Trigonal bipyramidal |
To empathise this molecule's properties, such every bit its reactivity, polarity, and more, one needs to know the SF4 Lewis structure offset.
SF4 Molecular Geometry
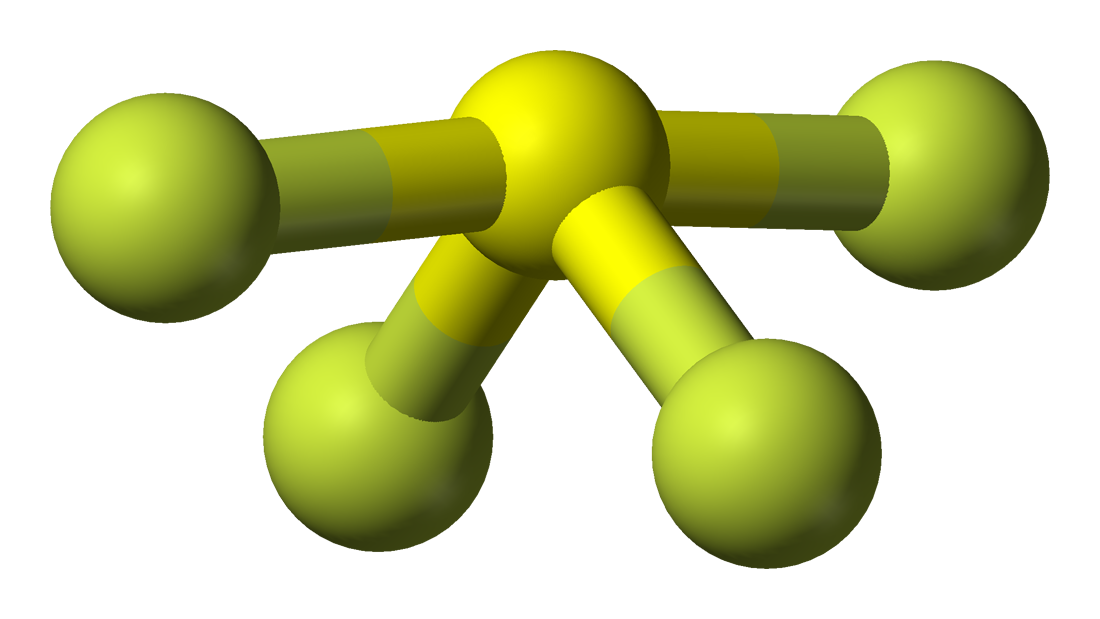
Information technology is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. A molecular formula helps to know the exact number and blazon of atoms nowadays in the given compound. Here at that place is one sulfur atom and four fluorine atoms in the compound, which makes information technology similar to the molecular formula of AX4E.
Molecules having a molecular formula of AX4E have trigonal bipyramidal molecular geometry. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. As there is one lonely pair on the central atom, it repels the bonding pair of electrons, which tweaks the shape a little bit and makes information technology announced like a see-saw. The electrons follow this blueprint of arrangement post-obit the VSEPR rule to minimize the repulsion forces between the lone pairs of electrons to maximize the molecule'south stability.
Hence, SF4 has a trigonal bipyramidal molecular geometry.
SF4 Lewis Construction
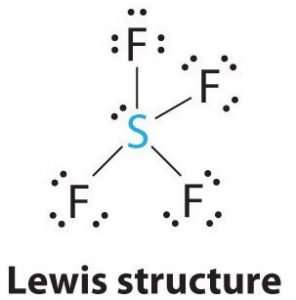
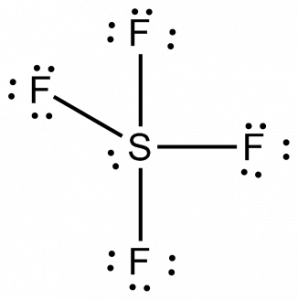
Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. The valence electrons that participate in forming bonds are called bonding pairs of electrons, whereas the electrons that practise not participate or form whatever bonds are chosen nonbonding pairs of electrons or alone pairs.
And to depict the Lewis structure of SF4, we first need to know the total number of valence electrons in this molecule.
Every bit one can probably run into, there is i sulfur atom in this compound and four fluorine atoms. To know the total valence electrons of this compound, nosotros need to know the valence electrons of both the atoms individually.
- Valence electrons of Sulfur: 6
- Valence electrons of Fluorine: 4* (7)
( as there are four fluorine atoms, we have to consider valence electrons of all atoms)
Total number of valence electrons in SF4 = number of valence electrons in sulfur + number of valence electrons in fluorine
= six + 28
= 34 valence electrons
Now that we know the full number of valence electrons, information technology would become like shooting fish in a barrel for the states to understand the bond germination between the atoms and the complete arrangement of the molecule too.
Sulfur will be the central atom in this molecule as it is the to the lowest degree electronegative, with four fluorine atoms forming bonds on the sides of this central cantlet. Every fluorine cantlet volition form a bond with the key cantlet, which ways there volition be 4 bonds in the molecule construction using up four valence electrons of fluorine atoms and 4 electrons of the sulfur atom. So at present, viii valence electrons are used, reducing the number of valence electrons from 34 to 24. All the fluorine atoms take six valence electrons, and the central cantlet has two valence electrons.
Draw lines between S and F to evidence bonds and for lone pairs of electrons, use dots. Each fluorine atom will accept three pairs of 6 valence electrons ( shown as dots) on the atom, along with one bond with sulfur. In contrast, the central atom volition accept two valence electrons and four bonds.
Hence, the cardinal atom, sulfur, will take ane lone pair of electrons and four bonding pairs of electrons in the Lewis construction of SF4. At the aforementioned time, each fluorine cantlet will accept three solitary pairs.
Is SF4 polar?
Once we know the Lewis structure and molecular geometry of the given compound, it becomes easier to depict the molecule'south polarity. Here, one lone pair on the cardinal sulfur atom and four bonding pairs of electrons leads to the disproportionate distribution of electrons on the central atom.
Besides, equally the shape of the molecule is like a see-saw, ii fluorine atoms can abolish out each other's dipole moment, but the residual ii tin can't due to the electrons' arrangement. And as fluorine atoms are more electronegative than the sulfur cantlet, it results in uneven distribution of the charge. Hence the dipole moment is non canceled, which makes the molecule polar. So yes, SF4 is polar.
SF4 Hybridization
To know the hybridization of the SF4 molecule, let united states first expect at the regions of electron density for the cardinal atom.
Sulfur has four bonding pairs of electrons and one lone pair, making its total number of regions for electron density 5. Hence the sulfur atom uses 5 hybridized orbitals, one 3s orbital, 3 3p orbitals, and one 3d orbital. This organization of electrons around the atom and hybridized orbitals leads to the sp3d hybridization. One can also use the steric number to know the hybridization; here, the steric number is 5 for the sulfur atom.
Thus SF4 has sp3d hybridization.
SF4 Bond angles and shape
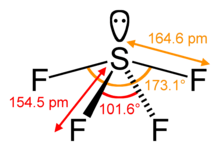
The primal sulfur atom forms four bonds with the neighboring fluorine atoms and has ane alone pair of electrons. Fluorine atoms on the equatorial positions have the bond angles of 102 degrees, and the axial ones have 173 degrees, which are a little dissimilar than the trigonal bipyramidal molecular geometry leading to a see-saw shape.
The lone pair on the fundamental atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms.
Concluding Remarks
To conclude all the properties we can say that,
- Sulfur Tetrafluoride has 34 valence electrons, out of which it forms iv covalent bonds and i lone pair of electrons on the central atom in its Lewis structure.
- There are iii lone pairs on each fluorine atom.
- It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a trigonal bipyramidal molecular geometry.
- SF4 has sp3d hybridization and is polar in nature.
Sf4 Lewis Structure Molecular Geometry,
Source: https://geometryofmolecules.com/sf4-lewis-structure-polarity/
Posted by: cruzsapeate.blogspot.com


0 Response to "Sf4 Lewis Structure Molecular Geometry"
Post a Comment